Product type 4 – Codependent technologies
Page last updated: September 2016
Introduction
This section provides guidance on the preparation of a submission to the PBAC that involves codependent technologies.
What are codependent technologies?
Health technologies are codependent where the patient health outcomes related to the use of one health technology (eg a medicine) are improved by the use of another health technology (eg a pathology test or an imaging technology). The use of the technologies needs to be combined (either sequentially or simultaneously) to achieve or enhance the intended clinical effect of either technology. Therefore, the net clinical benefits of the joint use of the technologies, as distinct from the net clinical benefit of each technology in isolation, needs to be determined for a health technology assessment. The cost-effectiveness and financial implications of the joint use of the technologies are also considered as part of the reimbursement decision.
The most common example of a codependent technology is a medicine-test combination where a new medicine seeking listing on the PBS has a related pathology test that may help to determine the population group eligible for that medicine. The Medical Services Advisory Committee (MSAC) classifies such tests as ‘investigative medical services’.
An investigative medical service can have several purposes,54 of which the following are most likely to be relevant to codependent technologies:
- establishing a predisposition or estimating a prognosis
- identifying a patient as suitable for a therapeutic medical service by predicting a variation in the effect of the therapeutic medical service
- measuring an early treatment effect on a surrogate outcome as the basis for predicting the extent of a later treatment effect on more patient-relevant outcomes
- monitoring a patient over time after an initial investigation to guide subsequent treatment decisions if the service needs to be repeated.
To achieve an improvement in health outcomes, the investigative information from the test must result in a change in the management of a subsequent therapeutic service. In this sense, the test can only indirectly improve health outcomes and any improvement also needs to be balanced against any harm that the service might cause. This purpose defines the need for a codependent technology to be assessed.
When is a codependent submission required?
A material codependency requiring a codependent submission arises when the Minister for Health requires advice from two different expert advisory committees because listing of the codependent technologies would involve two separate reimbursement schemes. For example, codependent technologies that require new listings or amendments to both the PBS and the Medicare Benefits Schedule (MBS) would need advice from both the PBAC and MSAC.
There are two different processes by which advice relating to the two reimbursement schemes can be formulated for the minister:
- integrated codependent submission – a combined submission for the two technologies is prepared and considered jointly by MSAC and the PBAC
- streamlined codependent submissions – individual submissions for each of the technologies (one for the test and one for the medicine) are lodged at the same time and are considered by MSAC and the PBAC, respectively, in parallel.
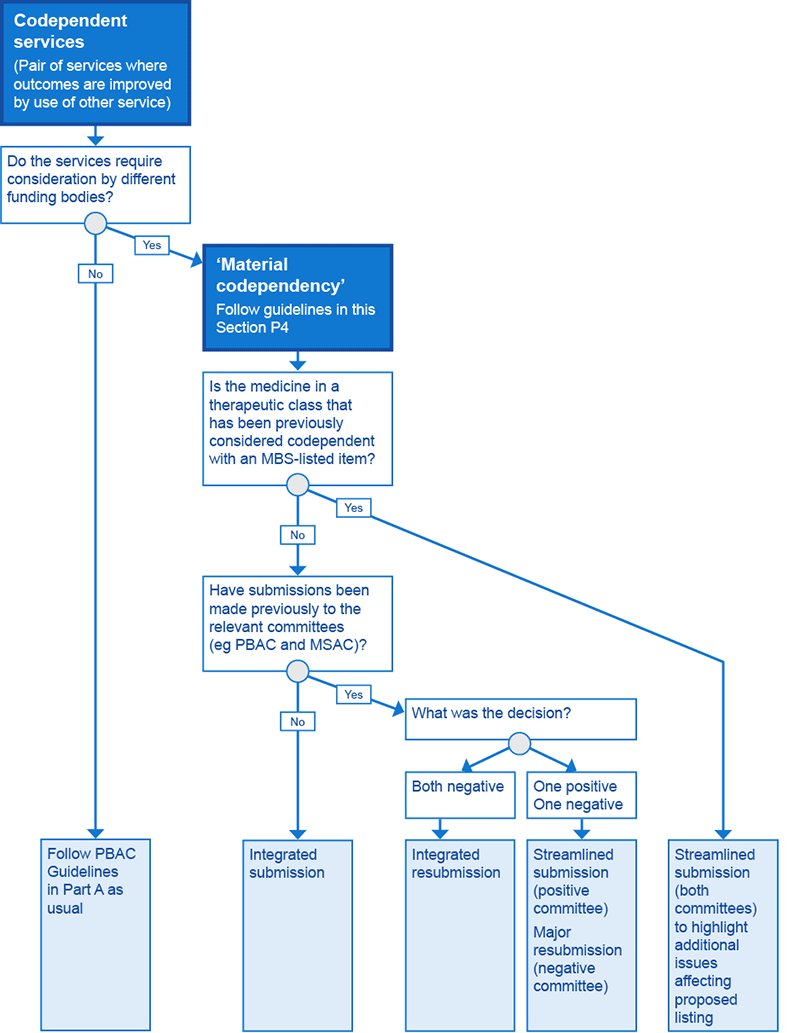
Further details of each process are provided below. Flowchart P4.1 shows the scenarios in which technologies with a material codependency have been considered for reimbursement by MSAC and the PBAC using an integrated or streamlined approach.
Integrated codependent submissions
Integrated submissions involve a submission of a medicine to the PBAC which also involves a codependent test or other investigative service that either:
- is not listed in the MBS; or
- requires a substantial amendment to the MBS to list it as intended, and thus entails joint consideration by both the PBAC and MSAC.
The format outlined in Subsection P4.2 is sufficient to meet the expectations of both the PBAC and MSAC with regard to a submission for PBS listing of the medicine and also a submission-based assessment for the MBS listing of the codependent pathology test or other investigative service.
In particular, lodge an integrated codependent submission when:
- the test and the medicine require a listing on the MBS and the PBS, respectively, and neither technology has been considered previously by either committee (MSAC or the PBAC)
- an integrated codependent resubmission is needed (ie both committees have indicated they are not satisfied with the information in the previous submission)
- the medicine is of a different therapeutic class to one that has been previously considered to be codependent with the MBS-listed companion test.
Streamlined codependent submissions
Codependent technologies can be efficiently reconsidered when, after previous consideration, only one committee has foreshadowed support for a technology in the pairing. For example, if MSAC has foreshadowed support for a codependent test or other investigative service, the lodgment of a resubmission to the PBAC may occur separately but in parallel to the lodgment of a streamlined resubmission to MSAC to ensure that MSAC’s advice is expeditiously aligned with the circumstances of any PBAC recommendation for the codependent medicine. Similarly, if an MBS item descriptor for a test or other investigative service needs minor amendment to accommodate access to a codependent medicine in the same therapeutic class as one that has been previously PBS listed, then a streamlined codependent submission to amend this item descriptor may be lodged with MSAC alongside the submission to the PBAC for the codependent medicine.
Flowchart P4.1 Classification of integrated and streamlined codependent submissions
MBS = Medicare Benefits Schedule; MSAC = Medical Services Advisory Committee; PBAC = Pharmaceutical Benefits Advisory Committee
Note: In the situation where the medicine is listed but the test is not, a material codependency does not exist because the decision to list the test falls to MSAC alone.


