Section 4 Use of the medicine in practice
Page last updated: September 2016
Introduction
Section 4 presents a set of budget impact analyses, and provides the most likely extent of use and financial estimates. These analyses are relevant to both the PBAC and the Australian Government. Section 4 is important for estimating the likely uptake of the proposed medicine in clinical practice and the cost impact on the Australian Government budget, and, in some cases, to negotiate risk-share arrangements.
Epidemiological and market-share analyses are the two broad approaches for developing utilisation and financial estimates, although their use is not mutually exclusive. An epidemiological approach is usually preferred for generating utilisation and financial estimates if the submission indicates a superior therapeutic conclusion in Subsection 2.8. However, a market-share approach might be preferred if the submission indicates a noninferior therapeutic conclusion in Subsection 2.8.
Justify the approach taken. Demonstrate concordance across both approaches where data inputs from one approach (epidemiological or market share) are uncertain.
Ensure that any estimates of the extent of use of the medicine (and other medicines and therapies) in the Australian setting are consistent with evidence presented throughout. Ensure that uptake of the medicine, change in the use of alternative medicines and offsets are all consistent with the clinical place of the proposed medicine (Section 1), the use of the medicine in the clinical trial setting (where applicable) (Section 2) and the circumstances presented in the economic evaluation (Section 3). Explain and justify any discrepancies.
Provide sufficient data in Section 4 so that the steps can be interpreted. Where the calculations used to generate estimates are not transparent in the main body of the submission, present additional data. The standardised Excel workbook for use with the epidemiological approach is available from the ‘Downloads’ section of the PBAC Guidelines website.
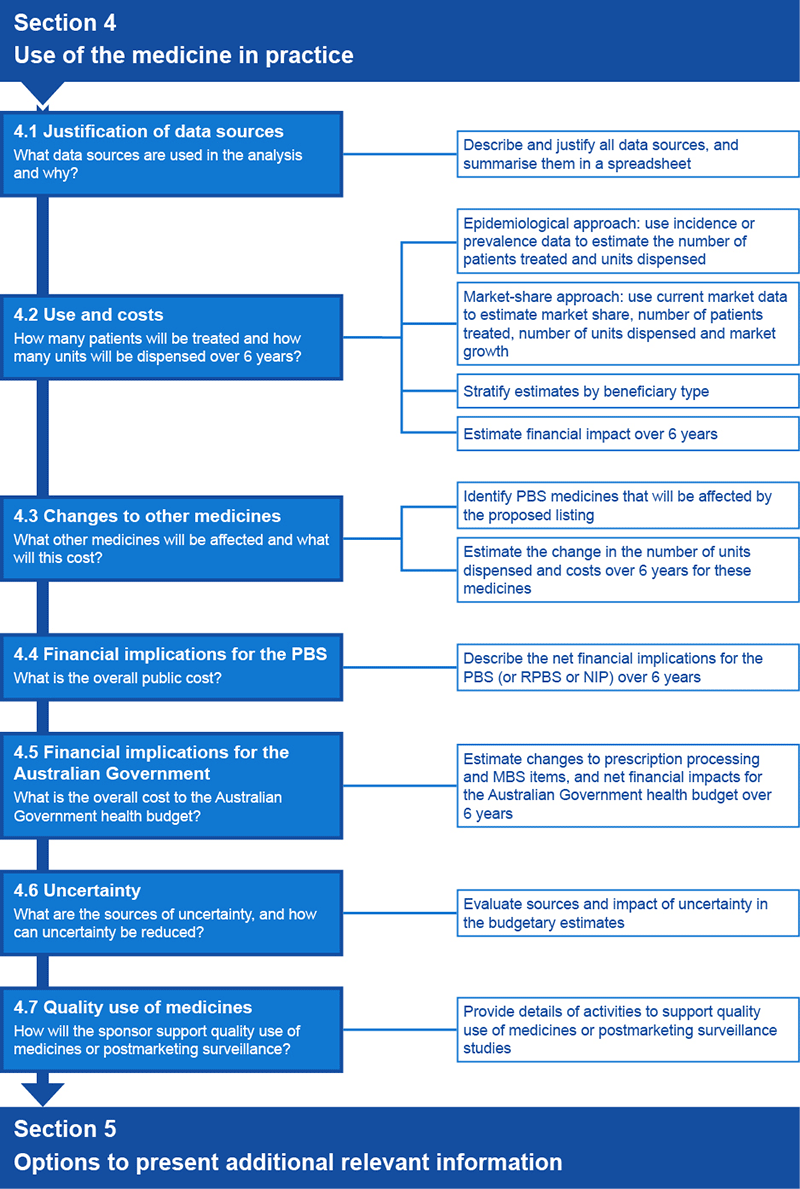
Flowchart 4.1 shows an overview of information requests in Section 4 for both the epidemiological and market-share approaches.
Flowchart 4.1 Overview of information requests for Section 4 of a submission to the PBAC
Epidemiological approach
An epidemiological approach estimates the number of people with the medical condition, and then estimates the use of the proposed medicine (see Subsection 4.2) and of other medicines (see Subsection 4.3) in the context of the patient group defined by the restriction or the main indication. Subsections 4.2–4.4 request financial analyses of health care resources subsidised through the relevant funding. Subsection 4.5 requests that these analyses be limited to include health care resources funded through the Australian Government health budget.
An epidemiological approach estimates the patients eligible for the proposed medicine; however, market-based data or market research may be required to establish estimates such as the rate of uptake of the medicine, the dose used in the community or the mix of beneficiary types.
In contrast to the economic evaluation presented in Section 3 of the submission, these financial analyses exclude health outcomes, do not use discounting, and exclude any resource item or copayment from a source other than the identified budget (see the relevant chapter of the Manual of resource items and their associated costs).
The epidemiological approach presented in Section 4 aligns with the utilisation and cost worksheets supplied alongside these guidelines, based on a standardised Excel workbook.
Where a submission seeks listing for more than one indication (see Subsection 1.4), present a separate standardised Excel workbook for each indication. As a final step in each of Subsections 4.4 and 4.5, aggregated these results across the indications.
Market-share approach
The market-share approach estimates the extent of the current market represented by the proposed patient indication and, consequently, the share likely to be taken by the proposed medicine. It is likely to be the most suitable approach where a medicine will completely substitute existing PBS-listed medicines.
In contrast with the epidemiological approach, the market-share approach allows an abbreviated presentation of information, where justified by an expectation of no market growth following listing, or provides an alternative way of generating estimates to compare with the epidemiological approach.
The key issue with estimates built on the market-share approach is whether the current market or market growth rate is expected to increase because of listing the proposed medicine on the PBS. If not, a medicine listed on a cost-minimisation basis would usually have a negligible effect on the net financial impact on the PBS, but may have financial impacts on other parts of the Australian Government health budget. Exceptions where medicines listed on a cost-minimisation basis may have net financial impact include:
- different MBS items required with the use of the new medicine – change in MBS costs
- different restriction level from the currently listed medicine – change in Services Australia (previously called DHS) costs.
In each of these circumstances, or if the proposed medicine is likely to increase the market size or its growth rate, it is critical to estimate the extent of this likely increase.
Quality use of medicines
Many factors influence the clinical outcome achieved by an individual after using a medicine. Identifying those factors and addressing them to achieve the quality use of the medicine is an important component of a therapeutic plan, including compliance with any requested restriction. Incorporate quality use of medicines (QUM) initiatives, where possible, throughout the estimates in Section 4. Summarise the activities that will be undertaken to promote QUM in Section 4.7.
Special pricing arrangements
Where a special pricing arrangement has been offered, include spreadsheets (using the supplied template) that show the costs over six years with and without the special pricing arrangement in place. Ensure that any comparators that also have a special pricing arrangement have their costs identified both with and without the special pricing arrangement. Carry the effect of the special pricing arrangement through the entire workbook.
Standardised Excel workbook
An Excel workbook developed for submissions is available at the PBAC Guidelines website, to guide sponsors on how to present the utilisation implications and financial implications for the PBS/RPBS, the MBS and Medicare. This workbook enables the PBAC to validate the presented estimates. Create additional spreadsheets to handle complex analyses or provide data to support assumptions, where required.
Ensure that the calculations flow through the spreadsheets, so that changes to any variable flow on to the results. To help understand the spreadsheets, apply clear and unambiguous labels to spreadsheet values, and cross-reference the data source (provide the data sources as an attachment). Provide clear and consistent formulas in the spreadsheets, to facilitate tracing and replicating the calculation flow.
Throughout Section 4, refer to the relevant spreadsheet number (eg Spreadsheet 1 of the standardised Excel workbook for PBAC submissions). Describe the approach, methods, assumptions and potential biases. Where possible, add comments to the Excel workbook to describe these factors, particularly if the approach is complex. Confidence in the estimates is reduced if the interpretation of calculations in the Excel workbook cannot be reconciled with the relevant assumptions or approach.
Copies of the data
To allow independent assessment of the data, attach copies of the data used (published, unpublished and commissioned). Ensure that the responses to Section 4 and the Excel workbook cross-reference the extraction of all data used to generate the estimates in these analyses from each attached data source (to the level of the page, table or figure number of each source document). Where commissioned data have been used, include the correspondence for the data request.


