Section 2 Clinical evaluation
Page last updated: September 2016
Introduction
In Section 2, present the best available clinical evidence to support the effectiveness and safety of the proposed medicine and patient indication (see Subsection 1.4).
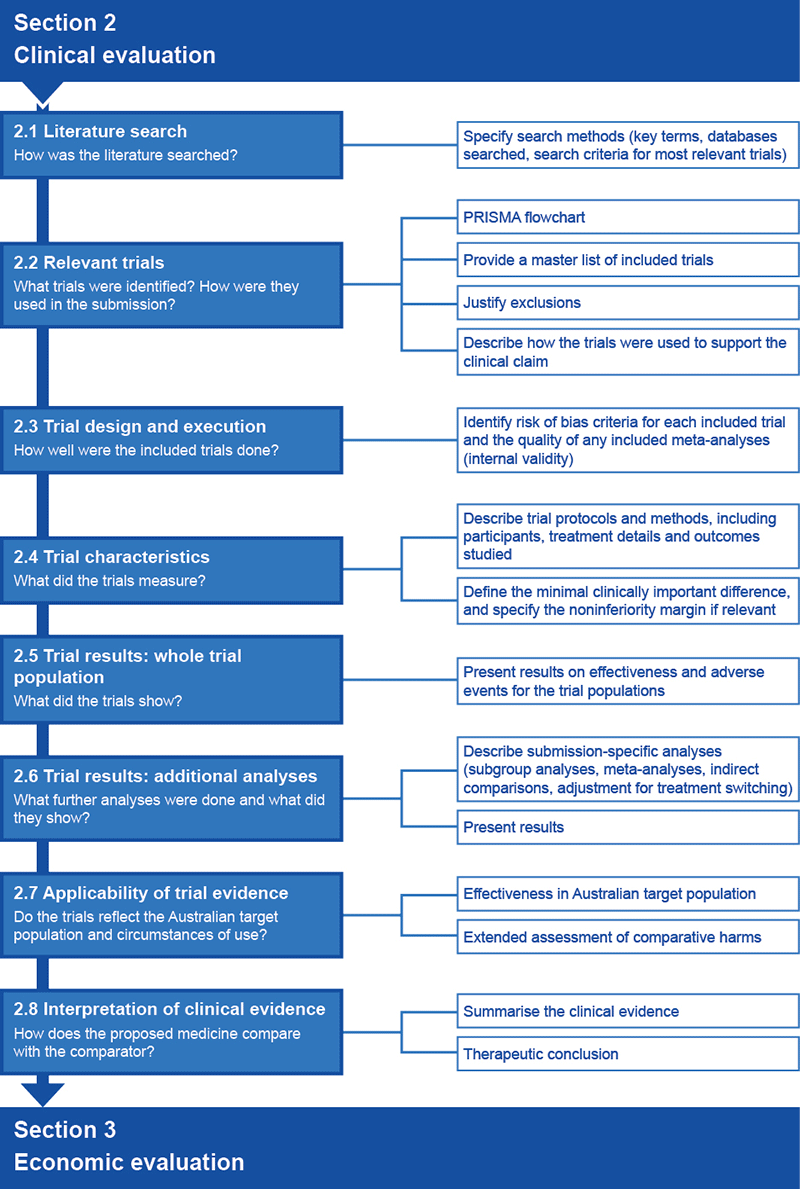
Section 2 has four components:
- a systematic search of the literature to identify relevant clinical trials or studies (Subsections 2.1–2.2)
- analysis and interpretation of the findings from each included trial, including trial-based estimates of the size of the treatment effect associated with the new medicine (or new use of the medicine) relative to the nominated comparator(s); factors that may influence an assessment of the credibility (internal validity) of the findings are also presented (Subsections 2.3–2.5)
- additional analyses that are used to estimate the comparative treatment effect of the new medicine (or new use of the medicine) when these cannot be derived from the whole trial population of trials presented in Section 2.5 (Subsection 2.6).
- an assessment of the applicability of the presented evidence to the Australian setting (Subsection 2.7).
A final subsection (Subsection 2.8) provides a therapeutic conclusion for the effectiveness and safety of the proposed medicine relative to the comparator. This conclusion forms the basis for the economic evaluation in Section 3.
The PBAC strongly prefers clinical and economic evaluations that are based on direct randomised trials. However, direct randomised trials are not always available, and these guidelines provide a framework for considering indirect comparisons of randomised trials and nonrandomised studies.
Flowchart 2.1 gives an overview of all the information requested for inclusion in Section 2.
Note: Unless otherwise specified, in the remainder of these guidelines, the term ‘trial’ includes both randomised trials (preferred) and nonrandomised studies.
Flowchart 2.1 Overview of information requests for Section 2 of a submission to the PBAC