2.1 Literature search methods
Page last updated: September 2016
Information Requests
- Define the criteria used to search for the most relevant evidence (Subsection 2.1.1)
- Tabulate the search terms (Subsection 2.1.1)
- Document the search strategy (Subsection 2.1.1)
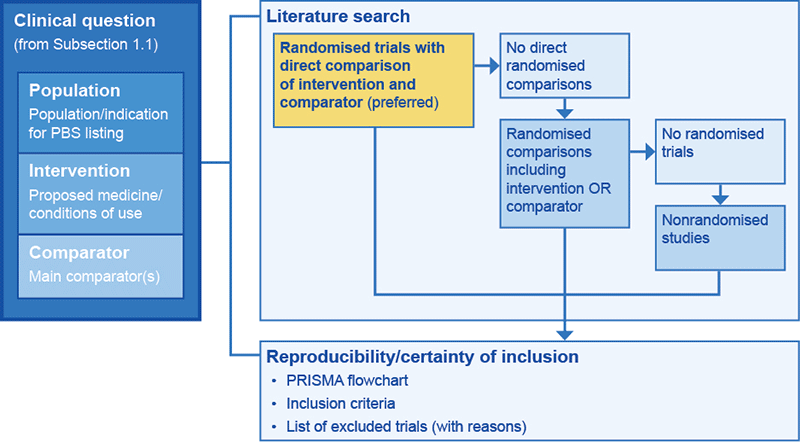
Subsection 2.1 details the search methods that ensure that all relevant randomised trials (or nonrandomised studies) have been included in the clinical evaluation. The primary objective is to identify all randomised trials that compare the proposed medicine with the main comparator. If no direct randomised comparisons are located, search for randomised trials that would enable an indirect comparison. If no indirect comparison is possible, search for nonrandomised studies.
This approach is based on an assumed hierarchy of evidence from randomised trials compared with nonrandomised studies. However, although direct randomised trials are typically less prone to bias than indirect comparisons or nonrandomised studies, it is not always true that indirect comparisons are less prone to bias than well-conducted nonrandomised studies. If you wish to present a well-conducted nonrandomised study alongside an indirect comparison of randomised trials, justify this approach.
An overview of this approach is shown in Flowchart 2.2.
Flowchart 2.2 Selection of trials for inclusion in the clinical evaluation
2.1.1 Search strategy
Present the search terms for the systematic literature search. Ensure that the search terms are consistent with the search criteria described in Appendix 2 and present them according to Table A2.1.
The primary objective of the literature search is to locate all randomised trials that, for the proposed patient indication, compare the proposed medicine directly with the main comparator for the target Australian population or a population that overlaps with the target Australian population.
If direct randomised trials comparing the proposed medicine with the main comparator are not identified, search again separately for randomised trials of either the proposed medicine or the main comparator. Present both search strategies (for the proposed medicine and for the main comparator). Use these trials to generate an indirect comparison.
If neither direct randomised trials nor other randomised trials suitable for an indirect comparison are retrieved, broaden the original search for the proposed medicine to identify all nonrandomised studies of the proposed medicine, preferably compared with the main comparator, that recruited participants whose characteristics overlap with the target population. Relevant study types include cohort studies, case-control studies and quasi-experimental studies.
In general, nonrandomised studies may provide useful information in the following situations:
- when it is unethical to conduct randomised trials (ie when the treatment effect is extraordinarily large in observational studies and so equipoise is not achieved)
- when randomised trials are not feasible (ie when the disease or condition is rare)
- when rare adverse events cannot be feasibly captured within the duration of a randomised trial (provide nonrandomised study data in addition to randomised trial data)
- when eligibility criteria for the trial are very restrictive, meaning that the applicability of the treatment effect to the target population is unknown (provide nonrandomised study data in addition to randomised trial data).
If the submission is based on nonrandomised studies, present both the search strategy for randomised trials and the search strategy for nonrandomised studies in Table A2.1 in Appendix 2 (provide detail in attachments).
Search the following sources:
- the published literature using the databases listed in Table A2.2 of Appendix 2
- registers of randomised trials
- the dossier seeking marketing approval submitted to the TGA, supplemented by checks with the sponsor’s head office and subsidiaries of the company (and any other original sponsor or colicensed companies) for any further randomised trials (which may be unpublished)
- reference lists of all relevant articles that are obtained.
Present the full search strategy for PubMed in an attachment. Summarise the search strategy for other data sources according to Table A2.2 (Appendix 2).


