P4.1 Overview of information requested in codependent submissions
Page last updated: September 2016
As already indicated, the amount of information to include in a codependent submission is contingent on any previous MSAC and PBAC consideration of public funding of the test and/or the medicine for the specific clinical condition under review.
Types of evidence
The approach to presenting evidence in an integrated codependent submission will differ according to whether direct evidence or linked evidence is available (see Figure P4.2 and Subsection P4.2 in Section 2 – Clinical evaluation).
Direct evidence
‘Direct evidence’ describes studies that compare groups of people receiving either the currently used diagnostic test/test strategy or the proposed diagnostic test/test strategy and measures the differential impact of the diagnostic method on patient health outcomes. If patients are randomised to receive the test, then biomarker status would be known and, on that basis, subsequent targeted therapy or usual care could be decided. If patients are randomised to not having the test, then a treatment would be received that is not targeted by the biomarker result.
Linked evidence
The ‘linked-evidence approach’ was proposed by MSAC whereby evidence of test accuracy comparing the proposed and current test/test strategy could be linked (if considered to be appropriately transferable) to separately sourced evidence of treatment effectiveness to approximate the likely clinical effectiveness of the proposed test/test strategy.
For example, this might involve linking evidence of the test’s performance (eg diagnostic accuracy) with evidence demonstrating that the test result changes the medicines or treatment prescribed, and with evidence that the alternative medicines have different effectiveness and safety profiles.
Integrated codependent submissions
If lodging an initial integrated codependent submission, consider addressing all the items outlined in Subsection P4.2. If lodging an integrated codependent resubmission, pay particular attention to the issues raised by both committees in deciding not to support the proposed codependent technologies.
The key to the evaluation of codependent technologies is to establish the basis of the codependency claim.55 Make the relationship between the test for the biomarker (investigative medical service) and the medicine explicit, particularly whether it is based on treatment effect modification and/or a prognostic effect. An integrated codependent submission must demonstrate that the medicine interacts with the biomarker that is identified by the test to improve patient health outcomes. That is, there must be an improved treatment effect because the medicine targets either an intrinsic property of the biomarker, or a physiological process for which the biomarker is a proxy.55
If the codependent technologies have evidence of both a background prognostic effect (tied to a specific biomarker) and treatment effect modification, present the net treatment effect (ie treatment effect modification controlling or adjusting for the background prognostic effect) in the submission.
Where a new codependent test and medicine targeting an as yet unproven biomarker are submitted for reimbursement, complete all information items in Subsection P4.2.
When a new biomarker(s) is proposed as part of a group of biomarkers in a submission, the aim is to gauge whether the addition of this new biomarker(s), when targeted by the medicine, results in further improvements in patient health outcomes. For a submission of this type, focus on the multiple biomarkers identified by a single test (rather than sequential testing). It is probable that the committees will have already addressed a codependent relationship between at least one of the biomarkers and the medicine and, in the interim, knowledge has advanced on how biomarkers work together to interact with the medicine. This scenario could encompass the possibility of a new or currently listed medicine, as well as a new or currently listed test. In this situation, all the items in Subsection P4.2 need to be addressed. Also seek advice from the Pharmaceutical Evaluation Branch on how to address these types of codependent technologies.
The preferred structure for an integrated codependent submission is given in Figures P4.2–P4.4. This is an adaptation of the preferred structure for submissions to the PBAC for medicines outlined in these guidelines.
Figure P4.2 Preferred structure of an integrated codependent submission for Section 1
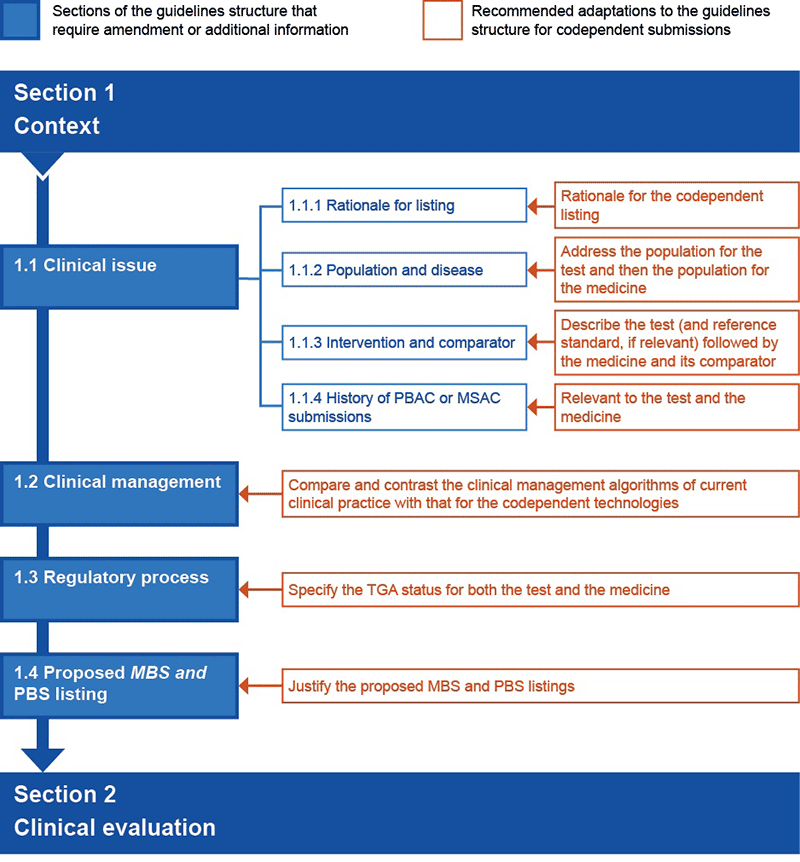
Figure P4.3 Preferred structure of an integrated codependent submission for Section 2
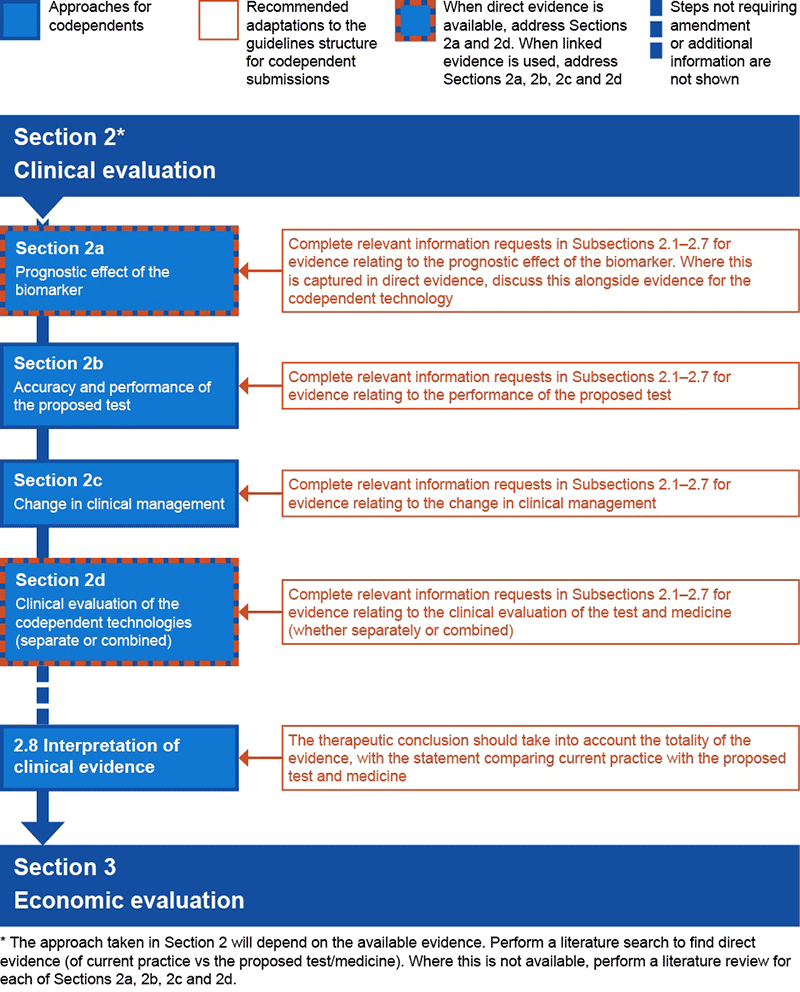
Figure P4.4 Preferred structure of an integrated codependent submission for
Sections 3 and 4
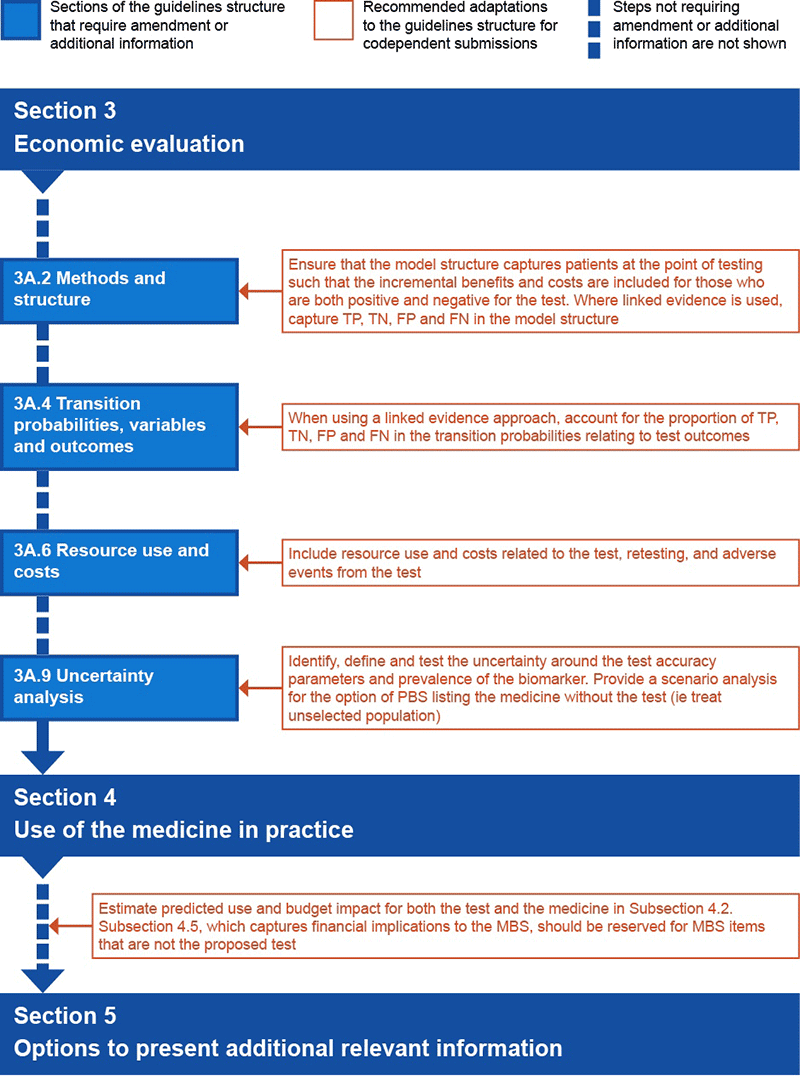
FN = false negative; FP = false positive; TN = true negative; TP = true positive
Streamlined codependent submissions
The principle of streamlined codependent submissions is to ensure that the substantive submission to seek the advice of one committee is coordinated with a less substantive submission to the other committee which has already signalled some support for the other codependent technology. In practice so far, the substantive submissions have had to be lodged with the PBAC, and streamlined submissions have been needed to ensure timely alignment of MSAC advice in the event of a PBAC recommendation.
Most experience has occurred where the PBAC has decided to defer or not to recommend a codependent medicine. In this circumstance, the purpose of the resubmission to the PBAC is to address the issues raised by the PBAC in reaching this outcome. There is also experience in the situation where the proposed medicine is in the same therapeutic class as a PBS-listed medicine, and listing is sought for essentially the same population, including with reference to patient eligibility being informed by biomarker test results. In this circumstance, the submission to the PBAC should address the items in Subsection P4.2 to the extent that is relevant, especially in relation to determining the individuals for inclusion in the proposed PBS restriction, in the submitted clinical evidence, and in the estimates of cost-effectiveness and financial implications.
Where a resubmission is being made to the PBAC, address the following matters in the streamlined resubmission to MSAC:
- a request to create or amend the MBS item for the proposed investigational medical service corresponding to the proposed medicine
- proposed wording for the MBS item descriptor (which should reflect the requested PBS restriction and the existing MBS item and/or the previous MSAC advice relevant to the proposed investigative medical service)
- a proposed MBS fee
- the costs to the MBS of the proposed listing (which should reflect the corresponding costs in the submission to the PBAC)
- a summary of the previous MSAC advice relevant to the proposed investigational medical service, and either
- confirming that the applicant agrees with each aspect of the advice (including, where appropriate, indicating how it has followed it in the submission to the PBAC and/or MSAC); or
- indicating where the applicant disagrees with any particular aspect of the advice, providing reasons (and an assessment of the consequences of adopting the applicant’s alternative approach rather than MSAC’s advice).
Where a submission is being made for a proposed medicine that is in the same therapeutic class as a PBS-listed medicine, and listing is sought for essentially the same population, including with reference to patient eligibility being informed by biomarker test results, address the first four of the matters listed above in the streamlined resubmission to MSAC. Also provide a detailed description of the testing strategy used in the trials presented in the submission to the PBAC, and the testing strategy for the corresponding trial of the comparator medicine – so that MSAC can assess any differences between these ‘evidentiary standards‘.


