Section 1 Context
Page last updated: September 2016
Introduction
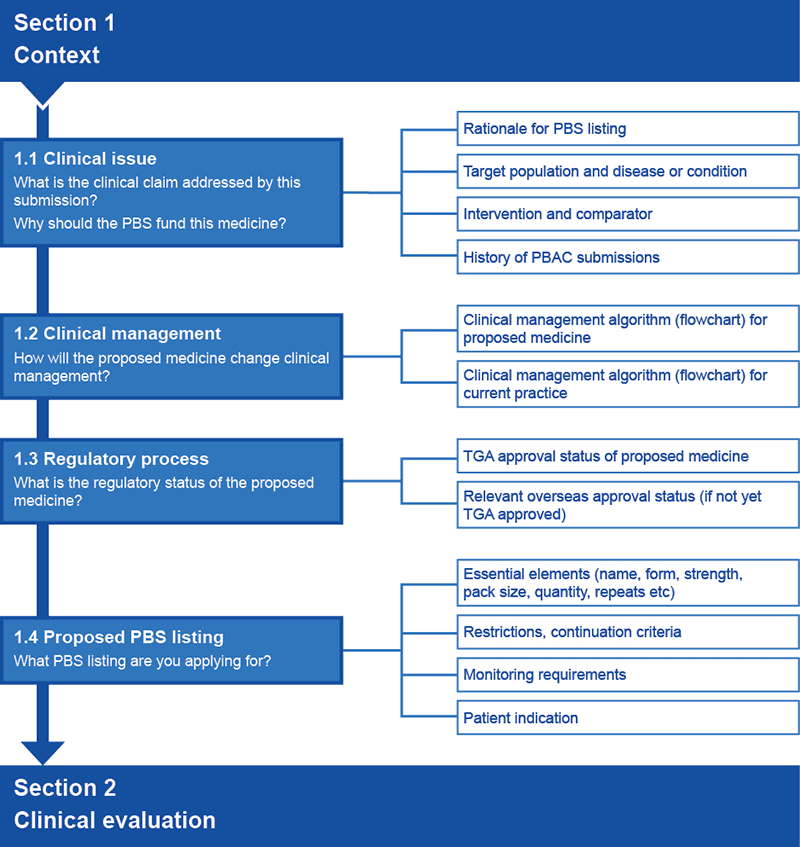
In Section 1, establish the context for the submission by providing the following essential details of the medicine and its proposed use:
- the rationale for listing key components of the clinical claim (Subsection 1.1)
- the way the proposed medicine will be used (Subsection 1.2)
- the regulatory status of the proposed medicine (Subsection 1.3)
- the proposed PBS listing (Subsection 1.4).
Flowchart 1.1 summarises the information requested for Section 1 of the submission.
Note: The singular term ‘comparator’ is used to denote one or more comparators. Provide all the requested information for each comparator, if there is more than one main comparator.
Flowchart 1.1 Overview of information requests for Section 1 of a submission to the PBAC