Section 3 – Economic evaluation
Page last updated: September 2016
The following section contains information requests for establishing the cost-effectiveness of the codependent technologies in terms of patient health outcomes.
Structure of the model
Additional Information Requests
- 36 (O) Indicate whether the model structure is consistent with other published economic evaluations in the same broad clinical management setting, initiating before the decision to test or treat
- 37 (O) Indicate whether the model structure is consistent with the clinical pathways provided in response to Item 7
- 38 (O) If relevant, provide a supplementary analysis of the nonhealth-related impacts associated with using the proposed test
36 (O) Consistency with other published economic evaluations
Include in Subsection 3A.2
Indicate whether the model structure is consistent with other published economic evaluations in the same broad clinical management setting, initiating before the decision to test or treat.
Indicate whether and why there are differences in model structure compared with the identified economic evaluations.
37 (O) Consistency with the clinical management pathways
Include in Subsection 3A.2
Indicate whether the model structure is consistent with the clinical pathways provided in response to Item 7. Indicate whether and why there are differences between the model structure and the clinical pathways, considering the following factors:
- The start point is testing of the eligible population (ie only a subset of the tested population goes forward to receive the proposed medicine). The less-preferred alternative is to start with the treatment and back-calculate the number (and costs) of testing the larger population.
- Where the model is constructed using a linked-evidence approach, include model arms to account for both accurate and inaccurate test results (see Items 39–42). This is not necessary if a single-randomised trial of the test is available (ie randomised to test versus no test trial arms) and only the evidentiary standard test is to be listed in the MBS – then the impact of inaccurate testing is incorporated in the health outcomes of the patients (this is analogous to a trial-based economic evaluation of the test and medicine pair). Where true positive, false positive, true negative and false negative test results are accounted for in the model, present a table specifying what source of estimates is used for each of the health outcomes and the health care resource provision in each of these four situations.
- A scenario analysis is provided where the proposed medicine is used without testing to show the extent of improvement in the incremental cost-effectiveness ratio (ICER) associated with using the test (see Items 7 and 58).
38 (O) Nonhealth-related impacts
Include in Subsection 3A.2
If relevant, provide a supplementary analysis of the nonhealth-related impacts associated with using the proposed test.
The same considerations for caregiver impact apply to codependent technologies as for other technologies, so the guidance provided in Part A of these guidelines will apply. The base-case economic model should be from a health system perspective. If other significant nonhealth impacts are expected, provide a supplementary analysis from a societal perspective. Discuss this in a supplementary analysis section. This could include the value to patients of being informed of their biomarker status.
Transition probabilities relating to test outcomes
Additional Information Requests
- If a linked-evidence approach was used in Section 2, calculate and include in the model:
- 39 (O) the positive predictive value (PPV) of the proposed test
- 40 (O) the complement of the PPV of the proposed test
- 41 (O) the negative predictive value (NPV) of the proposed test
- 42 (O) the complement of the NPV of the proposed test
- 43 (O) In the model, provide the incidence of adverse events associated with (i) the proposed medicine in patients with correct (true positive) and incorrect (false positive) positive test results, and (ii) the comparator medicine in patients with correct (true negative) and incorrect (false negative) negative test results; or (iii) reported from the direct evidence (ie in the circumstance that a double or single-randomised controlled trial of the test is available – analogous to a trial-based economic evaluation of the test/medicine pair)
- 44 (O) In the model, include the incidence of test-related adverse events for all those tested
- 45 (O) Where prognostic effect is operating in addition to treatment effect modification, ensure that the model adjusts for this factor when presenting absolute treatment effects
Calculate the following values for inclusion in the model using prevalence of the biomarker in the ‘tested’ population, and the sensitivity and specificity of the proposed test reported in Section 2:
- positive predictive value (PPV)
- negative predictive value (NPV)
- complement of PPV (1-PPV)
- complement of NPV (1-NPV).
39 (O) Positive predictive value of the proposed test
Include in Subsections 3A.4 and 3A.8
Calculating the PPV requires information on the sensitivity and specificity of the proposed test – as reported in the clinical evaluation section of the submission – and the prevalence (probability) of the biomarker in the target MBS population. It is the probability that a test positive result for the biomarker is correct. The PPV is used in a Bayesian manner to condition the model and calculate the transition probability associated with a true positive (use in Subsection 3A.4).

where SN = sensitivity, P = prevalence of the biomarker, SP = specificity
If agreement or concordance data are provided, rather than test accuracy data, measures such as the PPV cannot be accurately calculated since the subjects’ condition (as determined by a valid reference standard) is unknown. In this situation, a range of indicative PPVs (using a test nominated as the reference standard) might be used as transition probabilities and tested in sensitivity analyses. These analyses would explore the impact on the ICER of discrepancies in the agreement between the evidentiary standard test and other nominated reference standard tests that will be used in Australia to identify the biomarker.
40 (O) Complement of positive predictive value of the proposed test
Include in Subsections 3A.4 and 3A.8
One minus positive predictive value (1 – PPV) is the probability that a test positive result for the biomarker is incorrect (false positive). It predicts the consequence that patients will be treated unnecessarily, with a consequent decrement in expected treatment effectiveness and increment in harms. It is used in a Bayesian manner to condition the model and calculate transition probabilities.
If agreement or concordance data are provided rather than test accuracy data, present the complement of the range of indicative PPVs used to address Item 39.
41 (O) Negative predictive value of the proposed test
Include in Subsection 3A.4 and 3A.8
To calculate the NPV also requires information on the sensitivity and specificity of the proposed test – as reported in the clinical evaluation section of the submission – and the prevalence (probability) of the biomarker (eg phenotypic expression of mutation) in the target MBS population.
The NPV is the probability that a test negative result for the biomarker is correct. It is used in a Bayesian manner to condition the model and calculate transition probabilities.
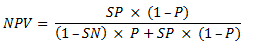
where SN = sensitivity, P = prevalence of the biomarker, SP = specificity
If agreement or concordance data are provided rather than test accuracy data, refer to guidance provided at Item 39.
42 (O) Complement of negative predictive value of the proposed test
Include in Subsection 3A.4 and 3A.8
One minus negative predictive value (1 – NPV) is the probability that a test negative is incorrect (false negative) and predicts the scenario where patients receive usual care instead of the proposed medicine with a consequent decrement in expected treatment effectiveness. It is used in a Bayesian manner to condition the model and calculate transition probabilities.
If agreement or concordance data are provided rather than test accuracy data, present the complement of the range of indicative NPVs used to address Item 41.
43 (O) Medicine-related adverse events in patients according to test result
Include in Subsection 2.5 or 2.6, and Section 3
In the model, provide the incidence of adverse events associated with (i) the proposed medicine in patients with correct (true positive) and incorrect (false positive) positive test results, and (ii) the comparator medicine in patients with correct (true negative) and incorrect (false negative) negative test results; or (iii) reported from the direct evidence (ie in the circumstance that a direct randomised trial of the test is available – analogous to a trial-based economic evaluation of the test–medicine pair).
Determine whether biomarker test status predicts or does not predict any comparative treatment effect variation in terms of adverse events (Subsection 2.5 or 2.6) and incorporate in the model (eg Subsections 3A.2 and 3A.4). Include the impact of medicine‐related adverse events on patients with a positive test result.
44 (O) Incidence of test-related adverse events
Include in Subsections 3A.4 and 3A.6
In the model, include the incidence of test‐related adverse events for all those tested. Refer to Items 16 and 29 (Subsection 3A.4). This includes adverse events from resampling to perform or reperform the test. Sometimes the original sample is not available or not of sufficient size to allow retesting, and a new sample is needed to reperform the test. Account for the costs associated with resampling in the model (Subsection 3A.6).
45 (O) Incorporation of net treatment effects (if relevant)
Include in Subsections 3A.2–3A.5
Where prognostic effect is operating in addition to treatment effect modification, ensure that the model adjusts for this factor when presenting absolute treatment effects.
Resource items and costs included in the model
Additional Information Requests
- Include the following costs in the model:
- 46 (O) unit test costs
- 47 (O) cost of sampling (if relevant)
- 48 (O) test administration costs
- 49 (O) costs of patient consultations with medical personnel regarding the test results and treatment planning
- 50 (O) costs of retesting and nonassessable results
- 51 (O) costs for adverse events associated with testing
- 52 (O) costs of additional and further testing as a result of the proposed test
- 53 (O) costs of medicine‐related adverse events, including those where the test result was false positive
- 54 (O) costs of other relevant health care resources (eg diagnostic, medical, hospital, allied health)
46 (O) Unit test costs
Include in Subsection 3A.6
In estimating the cost of testing, include the cost of tests undertaken on all patients for whom the medicine is being considered, not just the cost of the test for those who were found to be suitable for the medicine. Include all relevant sources of costs (eg infrastructure, training, quality assurance) that need to be captured in, and associated with, rendering an MBS-funded test (eg a pathology test).
47 (O) Cost of sampling (if relevant)
Include in Subsection 3A.6
For example, taking, storing, retrieving and transporting biopsy samples.
48 (O) Other relevant costs of test administration
Include in Subsection 3A.6
49 (O) Costs for patient consultations with medical personnel
Include in Subsection 3A.6
Include costs of patient consultations with medical personnel regarding the test results and treatment planning. Include an explanation as to the extent that these costs overlap with the already-occurring consultations for medical management.
50 (O) Costs of retesting and nonassessable results
Include in Subsection 3A.6
This could be covered at Item 46. In some cases, the test result is invalid or not assessable, and retesting of the sample is required. Ensure that any costs associated with retesting are in the model.
51 (O) Costs for adverse events associated with testing
Include in Subsection 3A.6
Provide costs for the items mentioned at Item 44.
52 (O) Costs of additional and further testing as a result of the proposed test
Include in Subsection 3A.6
This includes costs associated with any changes in subsequent types of testing for other purposes brought about by the use of the proposed test.
53 (O) Cost of medicine-related adverse events
Include in Subsection 3A.6
Provide costs for the items mentioned at Item 43. Include these costs in all arms of the model, including false positive test result arms.
54 (O) Costs of other relevant health care resources
Include in Subsection 3A.6.
For example, costs for diagnostic, medical, hospital and allied health resources.
Uncertainties in the model
Additional Information Requests
- 55 (O) Assess the uncertainty around the medicine’s therapeutic effectiveness
- 56 (O) If a linked-evidence approach was used in Section 2, assess the uncertainty around test accuracy
- 57 (O) If a linked-evidence approach was used in Section 2, assess the uncertainty around the prevalence of the biomarker
- 58 (O) If relevant, provide a scenario analysis for the option of PBS listing the medicine without the proposed test as a prerequisite
55 (O) Uncertainty around therapeutic effectiveness
Include in Subsection 3A.9
In instances where both treatment effect modification and prognostic effect are operating in the medicine-biomarker relationship, assess the uncertainty of the estimated incremental treatment effect and model this uncertainty.
56 (O) Uncertainty around test accuracy (if relevant)
Include in Subsection 3A.9
If a linked-evidence approach was used in Section 2, assess the uncertainty around test accuracy. In instances where there is heterogeneity in plausible test accuracy measures (sensitivity and specificity) in the collated evidence base, particularly for different eligible test options, vary these measures when calculating the PPV and NPV transition probabilities and assess the impact of this uncertainty on the estimated absolute treatment effect.
57 (O) Uncertainty around biomarker prevalence (if relevant)
Include in Subsection 3A.9
In instances where there is limited or heterogeneous information on the prevalence of the biomarker in the target MBS population, vary the plausible prevalence rate when calculating the PPV and NPV transition probabilities, and assess the impact of this uncertainty on the estimated absolute treatment effect.
58 (O) PBS listing the medicine without the biomarker test as a prerequisite
Include in Subsection 3A.9
Depending on the prevalence of the biomarker, in some cases there may be a net clinical benefit – which may be more cost-effective – to provide the medicine to patients without the use of biomarker testing. A scenario analysis should be used to make this explicit (see Item 7).


